
Shares of Aurobindo Pharma Ltd. declined over 6% on Friday after the manufacturing facility of a subsidiary received a warning letter from the US Food and Drug Administration.
A formulation manufacturing facility of Eugia Pharma Specialities Ltd., the company's subsidiary, received an official action-indicated status and warning letter from the US FDA. There is no impact on the existing supplies to US markets., the company said.
The agency uses the term OIA to refer to the recommendation of regulatory or administrative actions after an inspection.
Eugia Pharma Specialities specializes in general injectables and oncology, with a portfolio spanning various therapeutic areas.
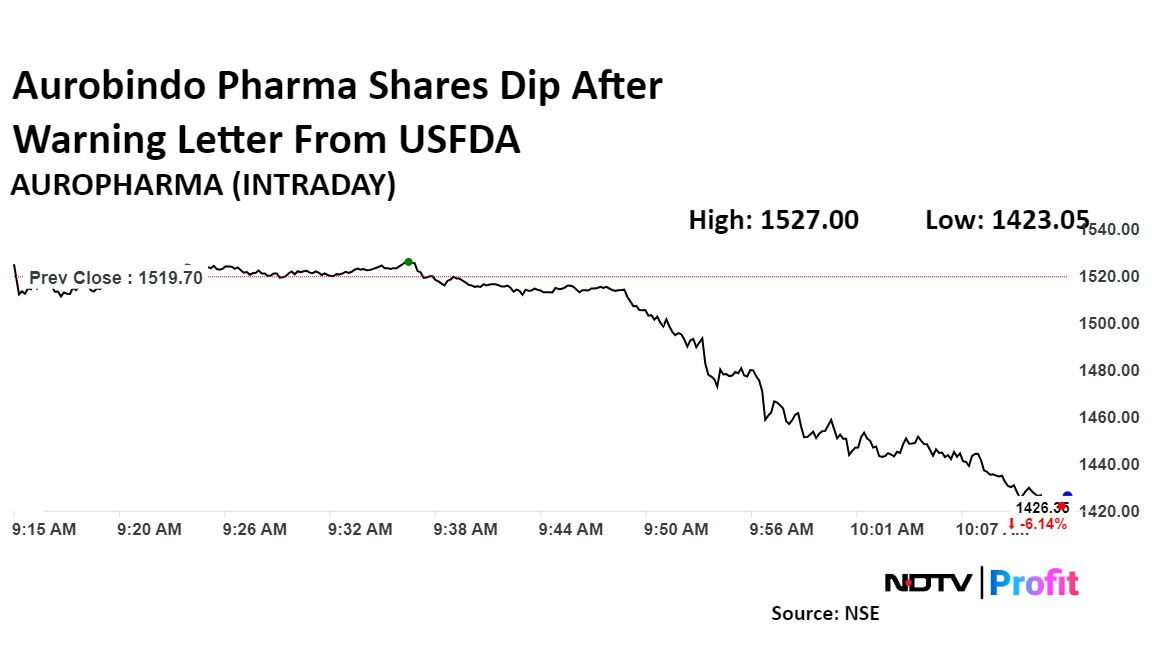
Shares of Aurobindo Pharma declined as much as 6.5%, before paring losses to trade 4% lower at Rs 1,460.8 apiece at 10:20 a.m., compared to 0.57% advance in the benchmark NSE Nifty 50.
The stock has gained 71% in the last 12 months and 34% on a year-to-date basis. The total traded volume so far in the day stood at 3 times its 30-day average. The relative strength index was at 60.
Twenty out of the 28 analysts tracking Aurobindo Pharma have a 'buy' rating on the stock, five recommend a 'hold' and three suggest a 'sell', according to Bloomberg data. The average of 12-month analyst price targets implies a potential downside of 8.4%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























