
Shares of Strides Pharma Science Ltd. jumped over 8% after its Singapore unit received U.S. drug regulatory approval for capsules used to reduce heart issues.
"The Icosapent Ethyl Capsules have a market size of $1.3 billion per IQVIA. The product will be manufactured at the company's facility in Bengaluru," according to an exchange filing on Sept. 23.
Icosapent Ethyl capsule is a prescription medication used in conjunction with other medicines like statins to reduce the risk of heart attack, stroke and heart issues in those afflicted with cardiovascular disease
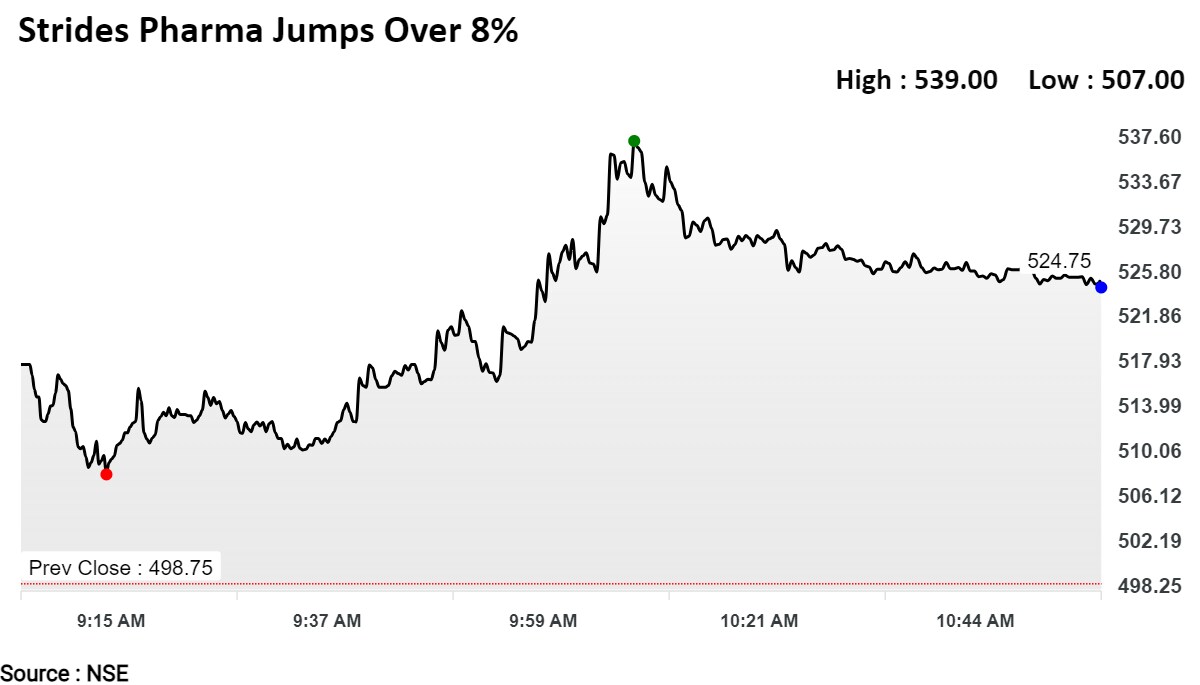
Shares of the pharma company rose as much as 8.07% to Rs 539. The stock is trading 6.07% higher at Rs 529 per share, compared to a 0.34% advance in the benchmark NSE Nifty 50 as of 11:29 a.m.
The stock has risen 51.02% on a year-to-date basis. The total traded volume so far in the day stood at 7.6 times its 30-day average. The relative strength index was at 75, suggesting the stock may be overbought.
Of the two analysts tracking the pharma company, one maintains a 'buy' while the other suggests a 'hold' on the stock, according to Bloomberg. The average of 12-month price targets given by analysts implies a potential downside of 0.3%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























