
Gland Pharma Ltd. has received tentative approval from the U.S. Food and Drug Administration for injection used to treat septic shock.
The pharmaceutical company received the tentative approval for angiotensin II 2.5 ml single-dose vial injection, the company said in an exchange filing on Wednesday.
The angiotensin II injection is used to increase blood pressure in adults with septic shock and other kind of shock.
Gland Pharma will launch the product with its marketing partner on receipt of final approval, it said.
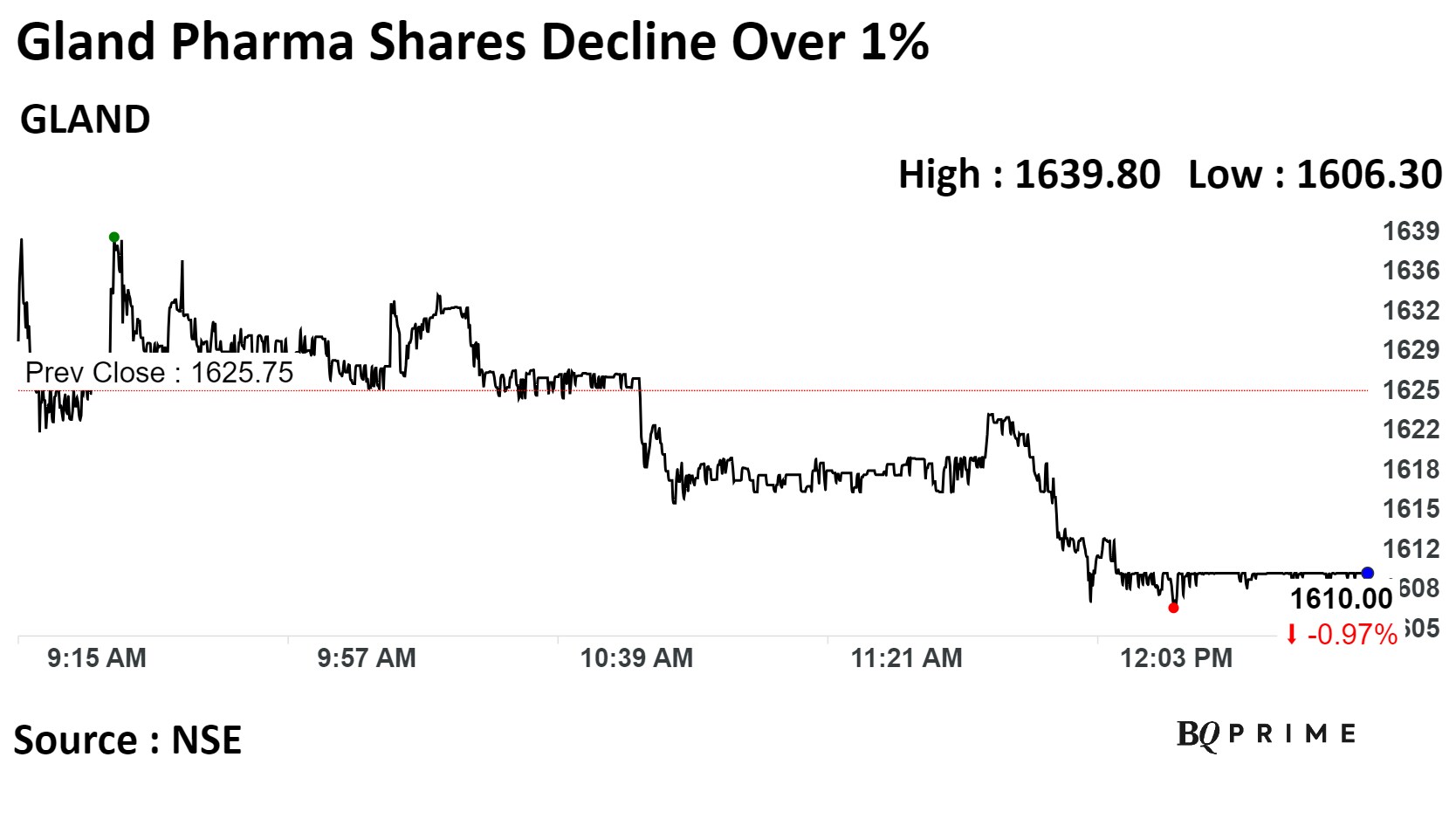
Shares of Gland Pharma were trading 0.97% lower at Rs 1,610 apiece, compared to a 0.99% advance in the benchmark NSE Nifty 50 as of 12:53 p.m.
It has risen 2.12% on a year-to-date basis. The total traded volume so far in the day stood at 30.9 times its 30-day average. The relative strength index was at 52.33.
Twelve out of 20 analysts tracking Gland Pharma maintain a 'buy' rating on the stock, three recommend a 'hold' and five suggest a 'sell', according to Bloomberg data. The average of 12-month analysts price target implies a potential downside of 6.7%
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























