
Alembic Pharmaceuticals Ltd.'s shares surged over 12% to a nearly six-month high after the company received final approval from the US regulator for its new drug application to treat ovarian cancer and other ailments.
The US Food & Drug Administration approved the abbreviated new drug application for Doxorubicin Hydrochloride Liposome injection and single-dose vials, as per a regulatory filing.
The approved ANDA is therapeutically equivalent to those developed by Baxter Healthcare Corp.
Doxorubicin Hydrochloride Liposome injection is used for the treatment of ovarian cancer, AIDS-related Kaposi's Sarcoma, and Multiple Myeloma. These products have an estimated market size of $29 million for the financial year ending March 2025, according to IQVIA.
Alembic Pharma has a cumulative total of 224 ANDA approvals (201 final approvals and 23 tentative approvals) from the USFDA.
Alembic Pharma Share Price Movement
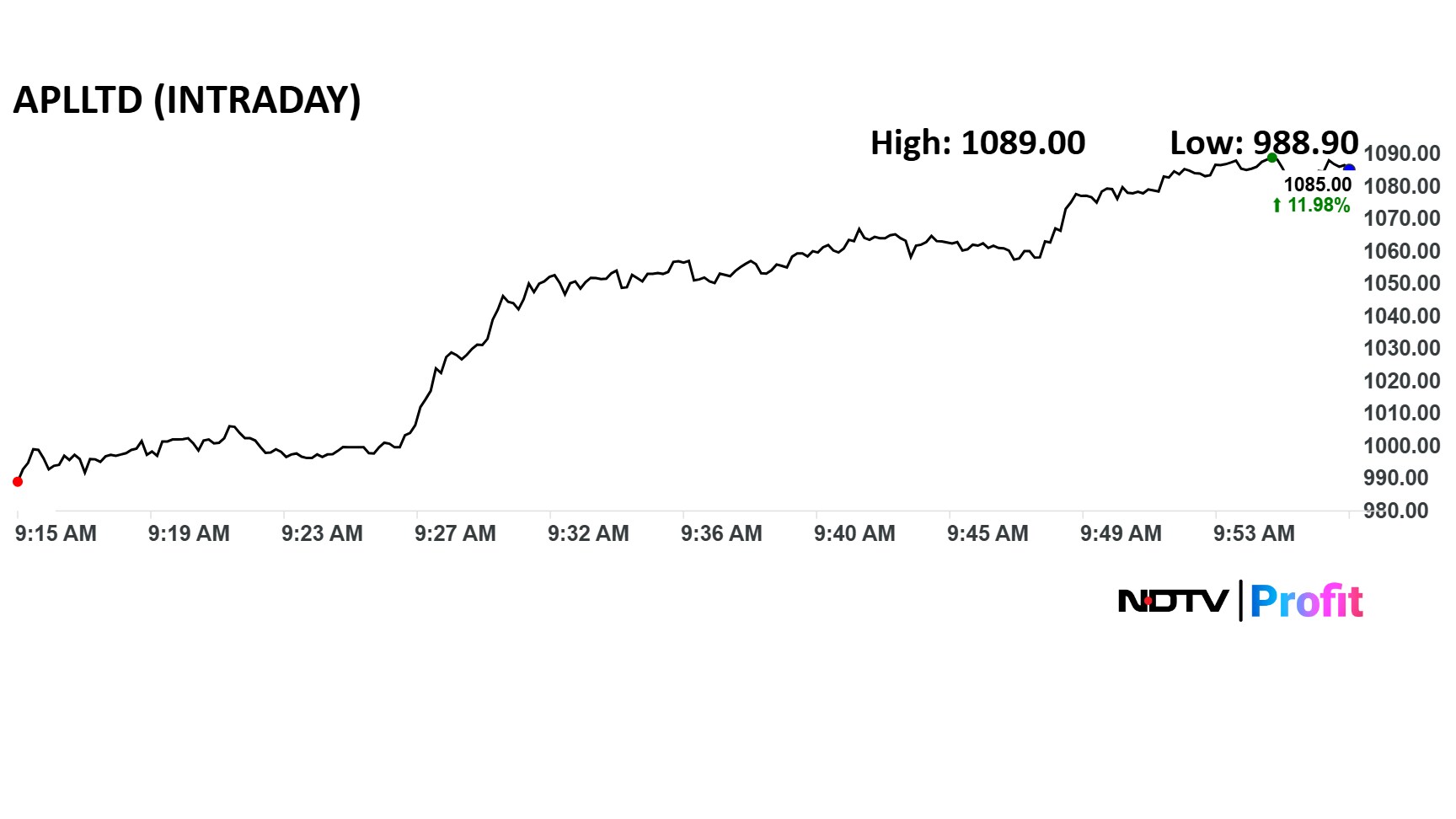
Alembic Pharma share price advanced over 12.4% intraday to Rs 1,089 apiece and traded close to that level as of 9:55 a.m. That was the highest since Jan. 7. The benchmark NSE Nifty 50 was down 0.26%.
The stock has risen 16.5% in the last 12 months. The total traded volume so far in the day stood at 51 times its 30-day average. The relative strength index was at 72.
Out of 13 analysts tracking Alembic Pharma, eight have a 'buy' rating on the stock, four recommend a 'hold' and one suggests a 'sell', according to Bloomberg data. The average of 12-month analysts' price target of Rs 1,011 implies a potential downside of 4.4%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























