
Shares of Sun Pharmaceutical Industries Ltd. fell over 4% on Friday to the lowest in over three weeks after it said that the US Food and Drug Administration has classified its Dadra facility as official action indicated. The FDA conducted inspection at the facility from Dec. 4 to Dec. 15, the drugmaker said in a statement to the exchanges, adding that it will cooperate with the US drug regulators to achieve a fully compliant status.
The "official action indicated" classification from the US drug regulator implies that the company will not be able to obtain FDA approval for future products until remedial actions are taken.
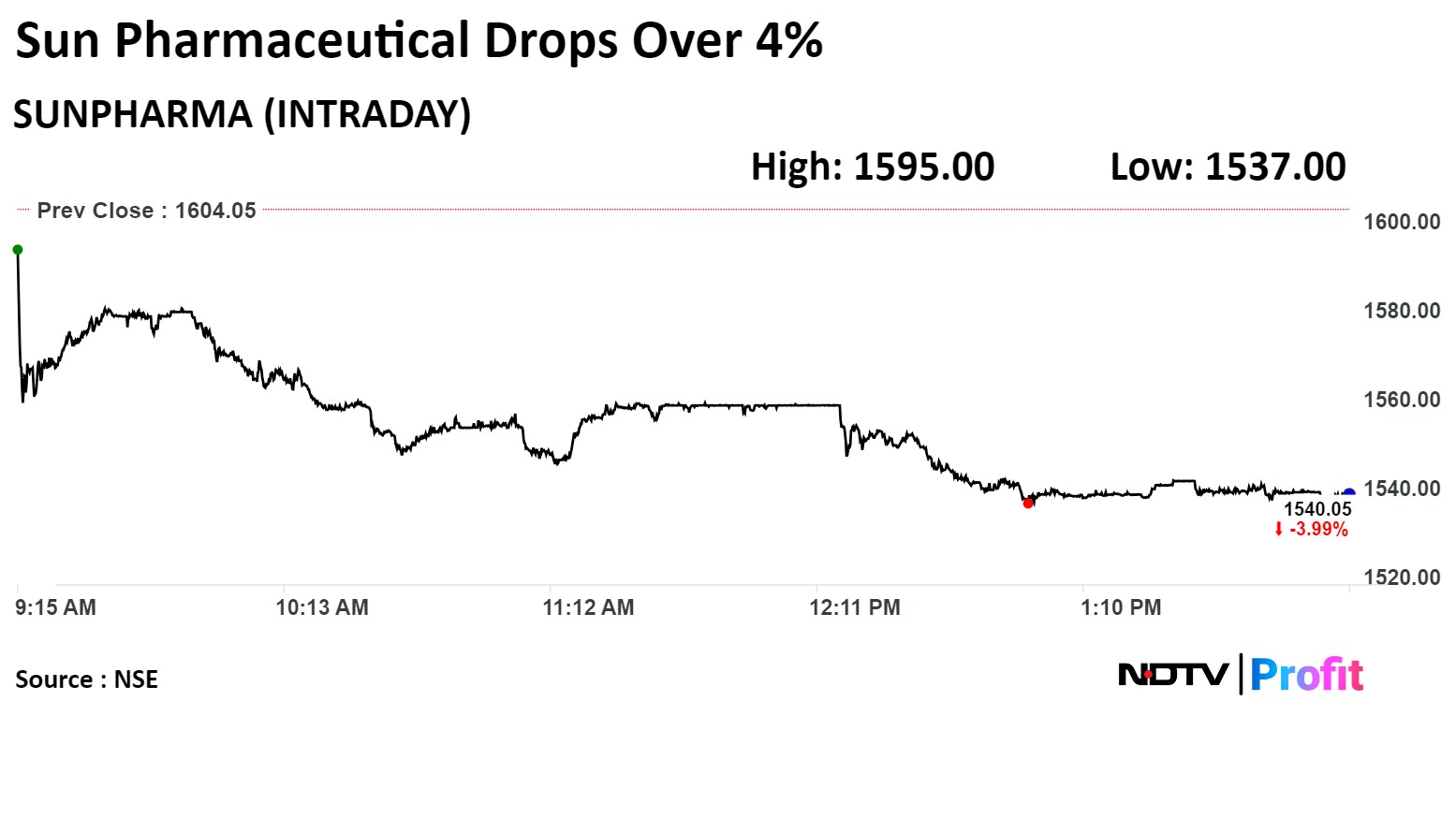
On the NSE, Sun Pharma's stock fell as much as 4.2% during the day to Rs 1,537 apiece, the lowest since March 20. It was trading 4.02% lower at Rs 1,539 per share, compared to a 0.84% decline in the benchmark Nifty 50 at 2:12 p.m.
The share price has risen 55.87% in the last 12 months and 22.28% on a year-to-date basis. The total traded volume so far in the day stood at 3.8 times its 30-day average. The relative strength index was at 39.40.
Thirty-four out of the 41 analysts tracking the company have a 'buy' rating on the stock, six recommend 'hold' and one suggests 'sell', according to Bloomberg data. The average of 12-month analyst price targets implies a potential upside of 2.6%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























