
Granules India Ltd. failed to adhere to safety measures and procedures for monitoring its building and equipment used in drug production at its Gagillapur facility, the US Food and Drug Administration has said.
The US drug authority had given a detailed explanation of the safety code violation at various stages of production in a warning letter issued to Granules India on Feb. 26.
There were violation of current good manufacturing practice regulations for finished pharmaceuticals, it said. The drug authority's investigators found contamination in multiple ducts of non-dedicated equipment, which are used in producing finished drug product at the company's Gagillapur facility. Filters were rendered ineffective due to inadequate cleaning and maintenance process.
Investors also discovered multiple residue, and microbial contamination from different drug production in the swab sample of high efficiency particulate air filters, the warning letter said.
Granules India also lacked enough documentation of sanitation process for ducts located in the section between HEPA filters and cleaning, the US FDA said. The drug authority found the Indian pharmaceutical company's response regarding this inadequate.
Investigators also pointed out bird feathers and excreta in the air handling unit area, especially on the air purification units, ducts, tanks, and on floors at the drug manufacturing facility. It raised serious concern about contamination.
Granules India failed to detail the root cause analysis and assess similar risk elsewhere. Further, nets which the pharma company installed still allow small insects inside the AHU, the drugs authority said.
Granules India's lack of oversight resulted in insufficient monitoring of the filter replacement process during routine maintenance, preventing the detection of potential filter failures, air leakage, and contamination risks from compromised HEPA filters and ducting. These concerns remained unaddressed in Granules India's response, the US FDA said.
The US drug regulator has asked Granules India to provide retrospective assessment of cleaning effectiveness and evaluation of cross-contamination hazards. It has also asked the pharma company to give details of identity of residues, equipment which is inadequately cleaned, and whether contaminated products are released for distribution.
A corrective and preventive action plan is also required along with appropriate remediations to facility's cleaning process among other reports.
On Feb. 28, Granules India told Indian exchanges that it had received a warning letter regarding its Gagillapur facility. The US drug regulator inspected the facility in August 2024. It will respond within stipulated timeframe and seek a meeting with US FDA to convey its compliance process and progress, the company had said in the exchange filing.
Granules India Share Price Today
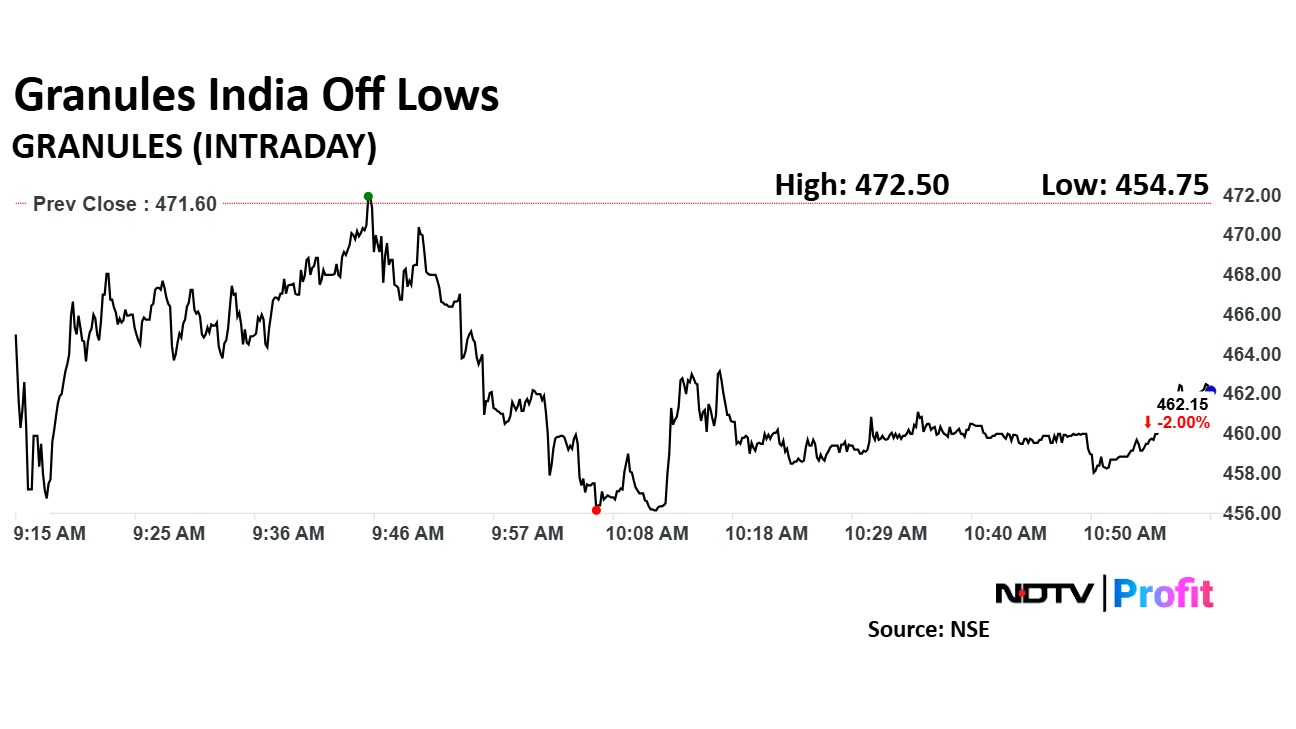
Granules India share price declined 3.57% to Rs 454.75 apiece. It was trading 2.10% down at Rs 461.70 apiece as of 10:59 a.m., as compared to a 0.92% advance in the NSE Nifty 50.
The stock declined 1.17% in 12 months. Total traded volume so far in the day stood at 4.4 times its 30-day average. The relative strength index was at 30.39.
Out of seven analysts tracking the company, six maintain a 'buy' rating, one recommends a 'hold', according to Bloomberg data. The average 12-month analysts' consensus price target implies an upside of 41.2%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























