
On Tuesday, Biocon Ltd. said its unit has received approval from the US health regulator for a genetic anti-bacterial medication. The approval was given to Biocon's unit in partnership with Carnegie Pharmaceuticals LLC
"This is to inform you that Biocon Pharma Ltd., a wholly owned subsidiary of the Company, in partnership with Carnegie Pharmaceuticals LLC, received tentative approval from the U.S. Food and Drug Administration (U.S. FDA) for the ANDA for Rifaximin Tablets, 550 mg," the company informed the exchanges.
Rifaximin tablets are indicated for reducing the risk of overt hepatic encephalopathy (HE) recurrence and to treat irritable bowel syndrome with diarrhoea (IBS-D) in adults.
Biocon's 4 global businesses include generics, biosimilars, research services and novel biologics, the company's official website said. The company also said that it is steadfastly investing in developing novel therapies for diabetes, oncology and immunology.
Earlier on Oct. 6, the company announced that its Board of Directors will meet on Nov. 11 to consider and approve the unaudited standalone and consolidated financial results for the quarter and half year ended September 30, 2025.
In line with the company's Code of Conduct for Prevention of Insider Trading and SEBI's Insider Trading Regulations, the trading window has been closed from October 1 to November 13, 2025, and will reopen on Friday, November 14, 2025.
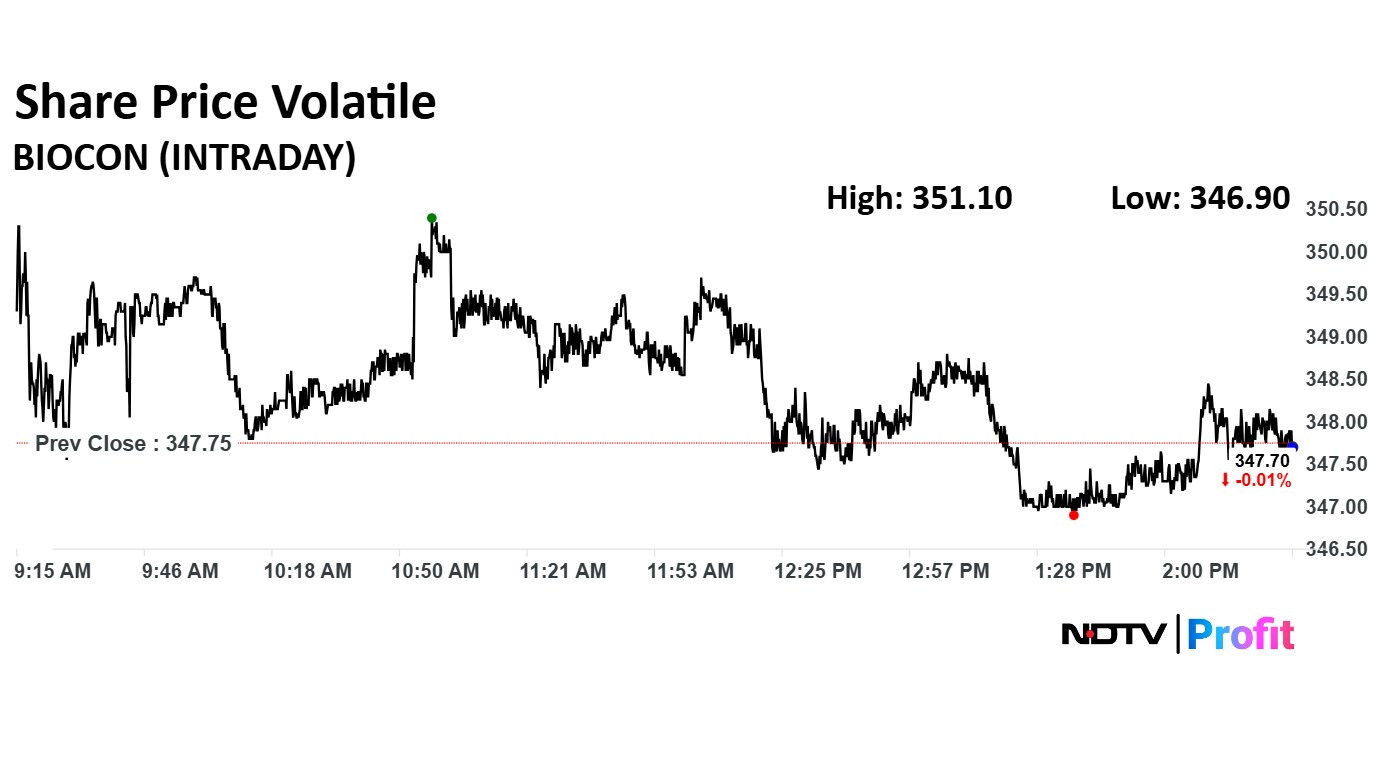
The scrip rose as much as 0.96% to Rs 351.10 apiece. It gave up gains to trade 0.13% lower at Rs 347.30 apiece, as of 02:03 p.m. This compares to a 0.50% advance in the NSE Nifty 50 Index.
It has risen 2.15% in the last 12 months. Total traded volume so far in the day stood at 0.44 times its 30-day average. The relative strength index was at 42.
Out of 18 analysts tracking the company, 10 maintain a 'buy' rating, three recommend a 'hold,' and five suggest 'sell,' according to Bloomberg data. The average 12-month consensus price target implies an upside of 10.3%
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























