
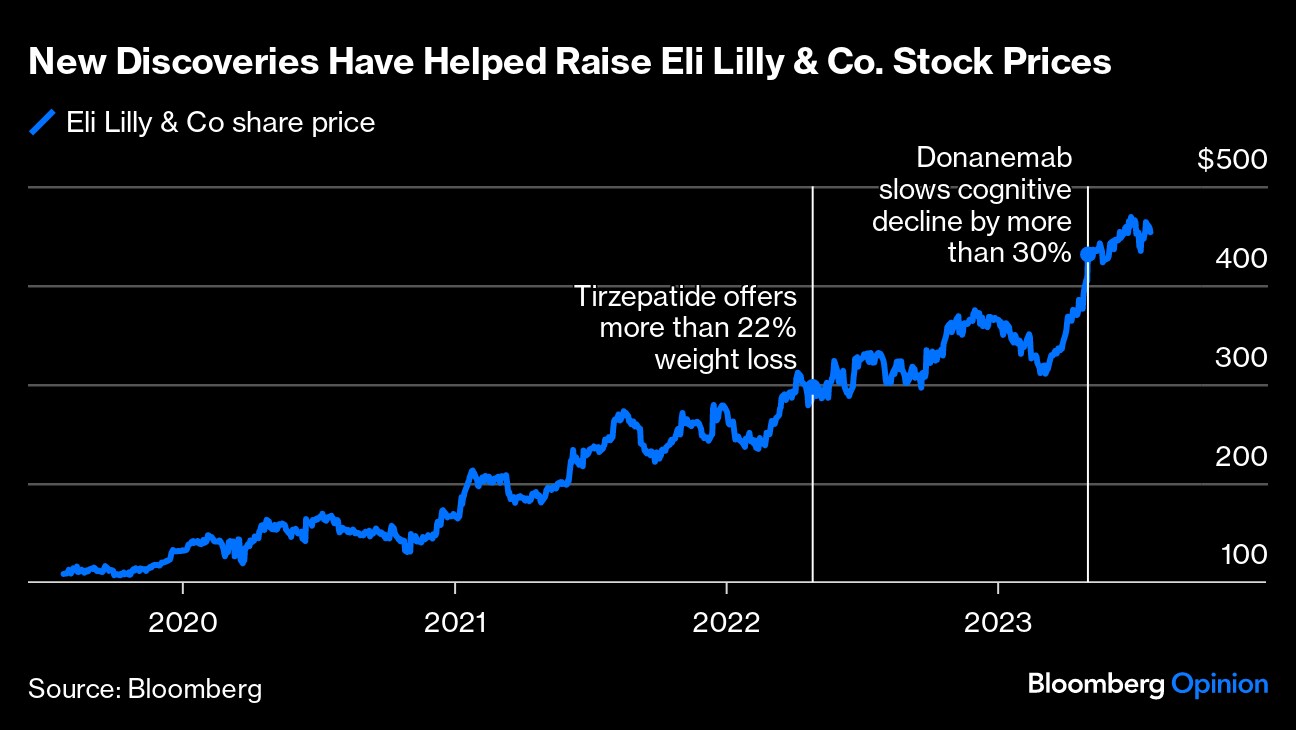
(Bloomberg Opinion) -- Two of the biggest stories in medicine this year — the arrival of the first disease-modifying treatments in Alzheimer's and the stunning transformations made possible by new weight-loss drugs — have something in common: Eli Lilly & Co.
By the end of 2023, the Indianapolis-based company is expected to get the Food and Drug Administration's nod for both its amyloid-targeting Alzheimer's treatment donanemab and the obesity drug tirzepatide, which it already markets for diabetes as Mounjaro. Both represent multi-billion-dollar opportunities for Lilly, and have propelled the company's stock steadily upward. A decade ago, it didn't even break the top 10 pharma companies based on its market cap; today, it often leads the pack.
To understand Lilly's R&D transformation and how it can build on those successes, I spoke with Daniel Skovronsky, the company's chief scientific and medical officer and president of Lilly Research Laboratories. Skovronsky came to the Indianpolis-based company through its 2010 acquisition of Avid Radiopharmaceuticals, which he'd founded to develop a PET scan that could detect amyloid plaques in the brains of people with Alzheimer's disease. Now, he needs to steer the company through something almost as difficult as failure: success.
LJ: Lilly is in the midst of an amazingly successful run. But those of us who follow the industry remember when the company's research organization was really struggling to make up for big patent losses — in fact, that wasn't even a decade ago. Things were so bad that the CEO at the time didn't take any pay, and tied people's bonuses to R&D output. You joined the company during that rocky period. What turned things around?
DS: We were in almost a vicious cycle of poor productivity. We kind of had to reinvent ourselves. I remember this moment, where the [former] CEO John C. Lechleiter said, “Here are some of the problems: The choices of what to work on haven't been great; And then when we do work on them, we're slower than others; And then when we do finish, they don't work. Those are things we need to fix.” Which is like almost everything there is in R&D.
Part of our thinking was how do we go fast but still generate adequate data to support reasonable success rates. Phase 3 failure rates were at one point well in excess of 50%, I think we were like 60 or 70% — it should be 60 or 70% are successful.
Changing our mentality to say that the unifying principle here is speed has made a big difference. We tried to move from a commercial pull model — what should we work on based on where the commercial presence is strongest — to a science push model, or what should we work on because the science is breaking and it looks great. So that's a really important shift. And then a higher level of rigor in decision making processes. It's always tempting to say, if this works, it'll be really, really big commercial opportunity. Let's do it, even if it's only a 5% chance of success. Of course, when we say that it's a 5% chance of success, we probably shouldn't fool ourselves — really, it's probably close to zero. We just can't be in a lottery business. We've got to pursue sound science.
What did that look like in practice for your core businesses?
We were committed to Alzheimer's disease. But we literally came up with a strategy, which I named Alzheimer's 2.0, because I felt like we needed a reboot or refresh on how we proceed Alzheimer's. Part of that strategy was that it was imperative to find a way to do Phase two clinical trials in Alzheimer's disease that could give us results that could then be reproduced in Phase 3.
It was a biomarker intensive approach. And it is still the only example of replication in Alzheimer's — where someone had something positive in phase two, and then they had it in Phase 3, which is a crazy thing to say, after decades of companies working on disease modifying drugs in Alzheimer's disease.
In diabetes, it was about moving beyond glucose control. It sounds obvious now, but at the time, glucose control was the key metric that that medicines got approved by and competed with each other [on]. We said, what are we really good at? We're really good at insulin and we're really good at incretins, let's work on the science there and see can we make radically better incretins [drugs like Mounjaro that mimic naturally-occuring hormones] or radically better insulin, so it could have very different benefits for patients. And so I just remember difficult meetings where we decided to-its hard to believe — but when we looked at the four therapeutic areas then, we were most concerned about our future in diabetes.
And so we rebooted our diabetes strategy and said those were our two main pillars. We've made great progress on incretins. Progress with insulins has been slower. Maybe we'll get there with insulins. We now have a weekly insulin in phase 3 and a glucose-sensing insulin that I hope will be in patients soon and I hope will be a big improvement.
I've been trying to think of another time in my career covering the pharma industry, other than during Covid, where there's been as much interest in a type of medicine as surround the GLP drugs. Was there a moment in the development of Mounjaro when you had an inkling you'd be swept into this kind of cultural zeitgeist?
I wish I could say yes, that I knew from the beginning. That's not how it worked.
We knew that obesity was this huge problem on the scale of a pandemic in the developed world, largely driven by genetic factors, which are then combined with an environment of sort of rich, readily available calories and obesogenic foods. And people with obesity have a much higher risk of heart disease of developing type Two Diabetes of cancer or dying from Covid, of rheumatoid arthritis and obstructive sleep apnea — like you name it and obesity is quite likely an important risk factor. So we wanted to work on that disease and you might think the commercial people should be super excited because it's a huge market. It's the opposite. They're like, diabetes is the market. Drugs for obesity have never been successful — let's not spend time there. It was really a science and medicine driven approach, not “this is going to become a viral internet sensation.” Probably the first time we saw it was when everyone else did, which was when Novo launched their drug and we launched ours [for diabetes].
Lilly recently offered data suggesting a newer obesity drug called retatrutide (also known as “Triple G”) could help some people lose as much as 30% of their body weight. As the bar for weight loss gets higher with each new drug, it's hard not to wonder about the end game. As Lilly and many others continue to develop newer types of weight loss drugs — is there a point after which there's no more need for improvement?
I showed a slide at a company meeting that showed the number of people in our tirzepatide obesity study who no longer had obesity, their BMI was below 30, at the end of the study. But then I showed the other half — they took this great drug and it's transformational efficacy, and they still have the condition we're trying to treat. And so we do need something stronger. Triple G could be that. But you can't keep doing this forever, right? We'll probably get the vast majority to their goal weights with a drug like Triple G. So how much effort should we make to make something that's stronger again?
If tirzepatide can offer 50%, and maybe Triple G will get us to three-quarters, does that mean that 10 years from now, if I look around the country or the world, there'll be a half as many people with obesity or a quarter as many people with obesity? I don't think so. It's not just about stronger medicines, there are other things that are needed.
One of the things that's needed is to generate more conversation and more evidence about the benefits of weight loss on different conditions. We're doing studies — a heart failure study, a sleep apnea study, and things like that — to show how obesity affects those diseases. Because when a doctor sees this as a disease and not just a societal problem, or cosmetic problem or willpower problem, then that'll level stimulate more conversations with patients and more appropriate usage of these drugs once they're approved.
Medicines need to fit into people's lives, that generally means they have to be easily and widely available, and easily used. And so is the next horizon making something even stronger? Or could we make something that's an oral pill…that could be more easily manufactured and more widely available and more easily used by patients? We start to think about medicines that could maybe reset your body weight and could have very long lasting effects-maybe once a year or something like that. That's dreamland stuff right now. But when we think about the pandemic of obesity, it's probably more ideas like that than just pursuing more weight loss.
It's taken decades to get to these first disease-modifying drugs in Alzheimer's. What's been learned along the way that could make this all go faster the next time?
Some of the things we learned we can apply to other diseases. I have my own biases, but to me the pivotal breakthrough in Alzheimer's disease over the last few decades has been the ability to have biomarkers, initially imaging and now blood based biomarkers, that can tell us the progression and pathology of the disease. That gives us something to aim at — we can know if our drug is working, we can know which patients to treat. Without them, Alzheimer's was so heterogeneous — and many of the patients clinically diagnosed with Alzheimer's don't even have the disease. And then some are going to progress quickly. And some are going to go slowly.
We don't have that for a lot of other prevalent diseases. We don't really have that for psychiatric diseases [or] diseases like lupus or rheumatoid arthritis. And so we struggle with issues around patient heterogeneity.
In Alzheimer's, it took a decade or two to make those biomarkers, and it took people who were like, I want to work on this. And some of that was academia, some of it was companies, some of it was public private partnerships. Those are the kinds of things we probably need to stimulate in another chronic diseases. I think even obesity must be a heterogeneous disease. But we don't really understand how to diagnose what are the different causes of obesity, genetic factors and other things in different patients, which could be another frontier of diabetes and obesity.
While donanemab and Eisai/Biogen's Leqembi are a good start, they aren't a cure. How is Lilly approaching building on these drugs to bring more meaningful change to the lives of people with Alzheimer's and their families?
So why do we only have 30% slowing of the disease — why can't we stop the disease 100%? And it's an unreasonable thing that very few diseases we stop 100%. But we want to be unreasonable here and offer even bigger effects. How do we do that? There's only two good ideas that I see. One is go earlier [in the disease]. And we're doing that, for sure. So we'll know that answer pretty soon.
The other is we probably need combination therapy in the symptomatic patients. That's a big open question: How do we do combination therapy? In our view, just being simple and boring here, tau is the other big pathology of Alzheimer's and so we want to block the spread of tau in patients. The easy paradigm to think about is to remove all their plaques and then give them an anti tau drug and see if that can slow the disease progression even further. Those are the kinds of things we'll be doing.
Thinking about where Lilly was a decade or so ago versus today, I'm wondering how you manage expectations around keeping up this pace of successes.
It's an overnight success ten years in the making. We have to stay really humble in this business because the science is hard.
When I took this job, I noticed that I'm the 10th head of R&D at Lilly in 100 years. So that means it's an average of 10 years per person. And then I started ticking off really big new chapters Lilly has written in medicine, starting with insulin. We can hope that every 10 years or so maybe we get one, but our history isn't that we've had one every 10 years. Maybe this decade we make it two. We're definitely writing a new chapter in the treatment of obesity and treatment of Alzheimer's disease.
I think we set expectations that these things are rare. You have worked for a long time to get something like this. And so along the way, we need lots of other things that also work and are great medicines. We're not that good at predicting which ones are going to be the ones that are in the front page of The New York Times or Bloomberg or wherever. So it's better to work on great drugs based on great science, and some of them will write new chapters in medical history and others will be important drugs that help a lot of people, and that's good, too.
More From Lisa Jarvis at Bloomberg Opinion:
- The Fight Against Alzheimer's Is Entering a Critical Phase
- Tornado at Pfizer Plant Exposes Fragility of Drug Supply Chain
- If You Never Got Sick From Covid, Thank Your Genes
(Corrects the spelling of Skovronsky's name in the third paragraph.)
This column does not necessarily reflect the opinion of the editorial board or Bloomberg LP and its owners.
Lisa Jarvis is a Bloomberg Opinion columnist covering biotech, health care and the pharmaceutical industry. Previously, she was executive editor of Chemical & Engineering News.
More stories like this are available on bloomberg.com/opinion
©2023 Bloomberg L.P.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.























