
Shares of Cipla Ltd. fell over 7% on Thursday after the U.S. drug regulator revealed details of a warning letter to it Pithampur facility in Madhya Pradesh.
The warning letter summarises violations of current good manufacturing practices regulations for finished pharmaceuticals and product quality defect complaints.
The U.S. FDA also pointed out similar issues with the Goa facilities of the company, which is under 'Official Action Indicated' status. "FDA has cited similar CGMP observations at other facilities in your company's network," the warning letter said.
It has asked the pharma company to take help from third party consultants to address the issues at the facilities.
"FDA may withhold approval of new applications or supplements, listing your firm as a drug manufacturer until any violations are completely addressed and we confirm your compliance with CGMP. We may re-inspect to verify that you have completed corrective actions to any violations."
The company had notified about the U.S. FDA warning through an exchange filing on Nov. 18. The routine CGMP at the facility was held between Feb. 6 and 7.
"The company will respond to the warning letter within the stipulated timelines and work closely with the U.S. FDA to address the concerns in a holistic and timely manner to ensure sustained compliance," it said.
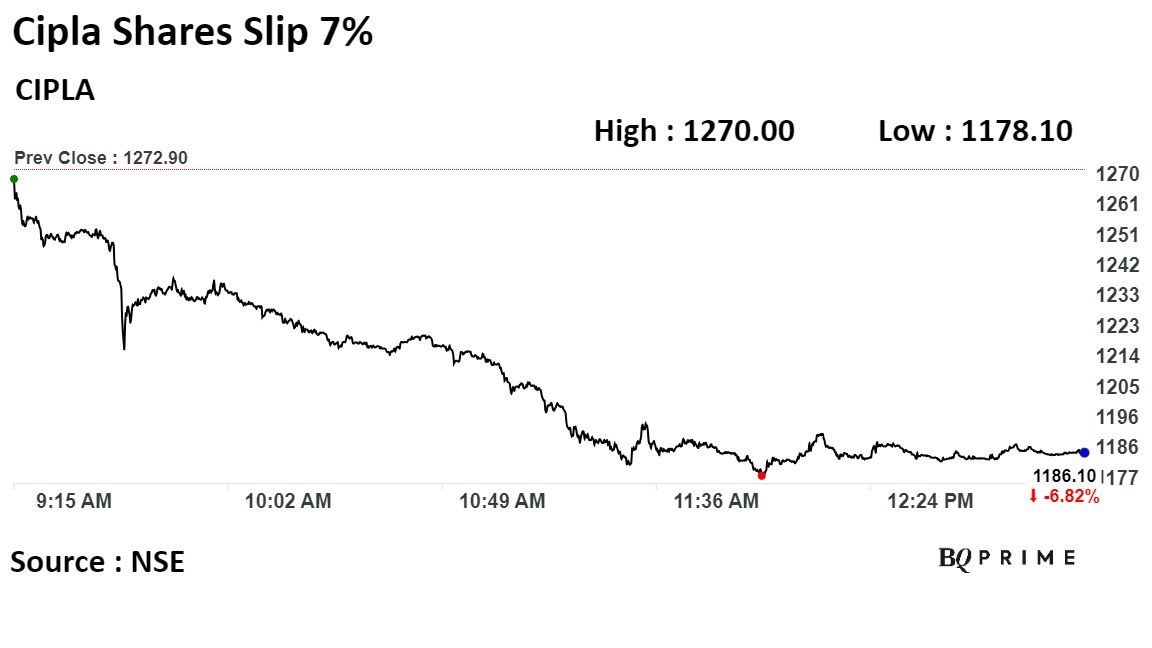
Shares of Cipla Ltd. fell as much as 7.35%, before paring loss to trade 6.76% lower at 1:05 p.m., compared to a 0.03% decline in the NSE Nifty 50.
The stock has risen 10.2% on a year-to-date basis. Total traded volume so far in the day stood at 5.5 times its 30-day average. The relative strength index was at 41.05.
Of the 40 analysts tracking the company, 30 maintain a 'buy' rating, seven recommend a 'hold,' and three suggest a 'sell', according to Bloomberg data. The average 12-month consensus price target implies an upside of 7.4%.
Essential Business Intelligence, Continuous LIVE TV, Sharp Market Insights, Practical Personal Finance Advice and Latest Stories — On NDTV Profit.




















